Heart chambers in a test tube: Using cardioids to understand heart development
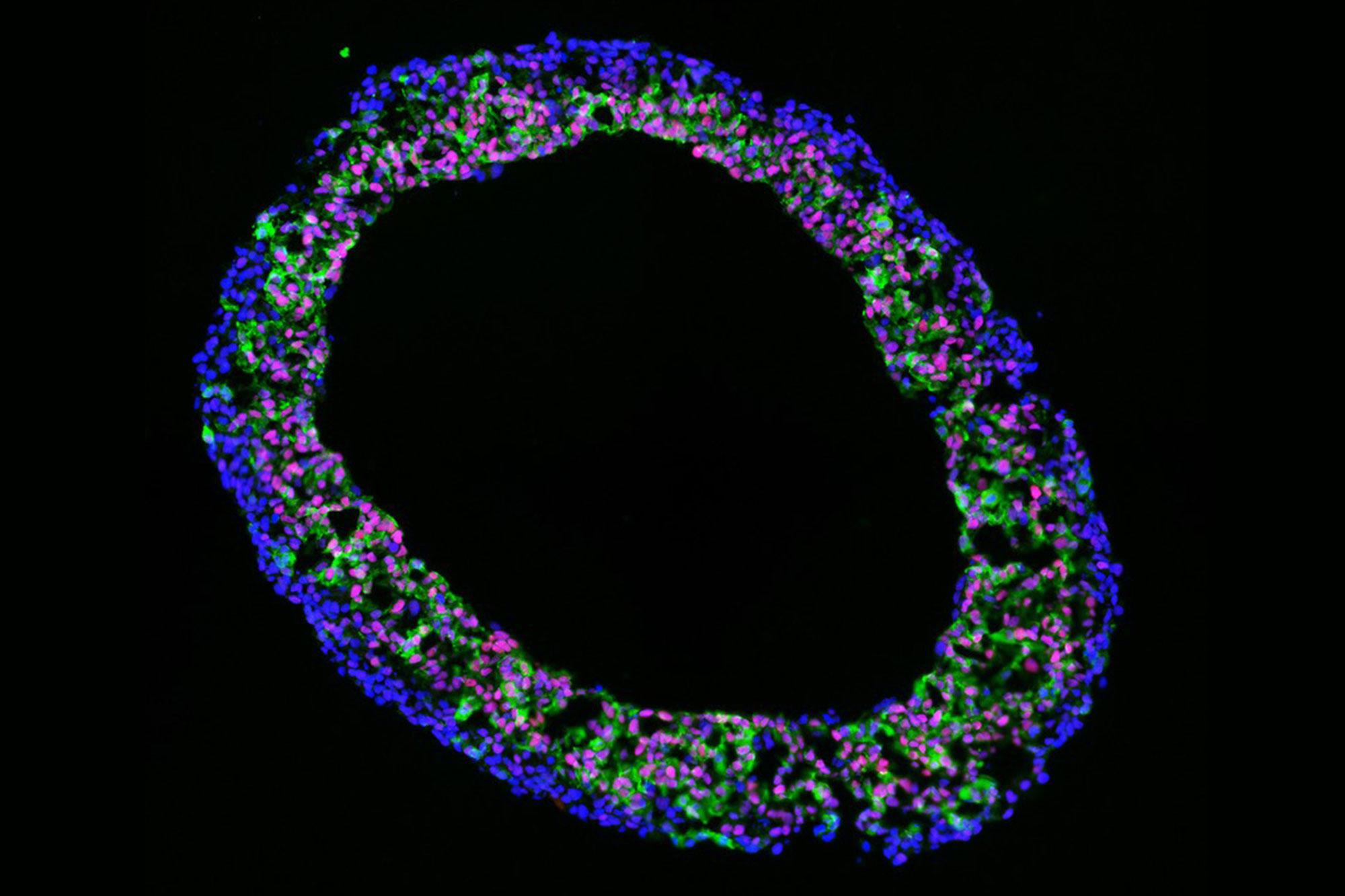
"Heart chamber organoids" from induced pluripotent stem cells provide new insights into human heart development in a test tube and offer an alternative to traditional research on mice.
Every year, around 600 to 800 children are born with congenital heart defects in Switzerland. To better understand the development of such diseases and advance new diagnoses and therapies, a University of Bern research team led by Marco Osterwalder is working on innovative models. Using human induced pluripotent stem cells, so-called "iPSCs", they are developing "heart organoids" – small, pulsating heart chambers in their lab. These artificial "cardioids" allow researchers to gain insights into human heart development in a test tube and offer an alternative that requires less animal testing compared to traditional research on mice.
In your opinion, what are the most important results and takeaways of your NRP 79 research project?
Based on a model originally developed by our project partner Sasha Mendjan in Vienna, we have successfully established cardioids in Bern. Cardioids are formed from cultures of induced pluripotent stem cells and form spherical structures that can be up to 2 milimeters in diameter. Genetically and functionally, cardioids demonstrate many similarities with embryonic heart chambers. For example, we can observe rhythmic contractions. We use the model to better understand how heart genes function and are regulated by gene switches, so-called enhancers. This allows us to investigate the mechanisms that may be responsible for congenital heart defects. So the question is: Which genes and sections of our genome play a decisive role in the development of heart defects?
A key finding is that many of the genes and specific enhancers that are active in the in vivo mouse heart are also active in the cardioid, which emphasises the relevance and transferability of the model for gene regulation. The model makes it possible to simplify genetic manipulations without the need for animal models, thereby simulating the effect of mutations on heart development in the lab. This provides important insights for future studies, especially relative to the mouse model. Several projects are currently underway. An additional focus is on characterising the function of heart genes and their control by enhancers in the organoid system.
What are the next steps in the project? Is the developed method already being used in practice, or are further developments necessary first?
The model is already being used for individual applications, for example to test genetic and epigenetic data. At the same time, a partner group in Vienna is working with large data sets and machine learning to improve predictions for heart development. These predictions can then be verified in heart organoids.
The existing model has already been enriched with mouse data. This will then form the basis for a forthcoming publication and for the development of a "map" of heart development control for transfer to humans. Additional data sources are increasingly becoming available internationally, including information from human embryonic hearts, which can be used to improve predictions in the future.
What are the advantages of the model for patient care?
This new method has great potential for the diagnosis and treatment of heart disease in patients: Genetic diagnoses can now be used to recognise mutations in patients with an increased risk of a heart defect – although it is often not known which of these are actually problematic. This is where our model comes into play: body cells could first be generated from the patient's skin cells, from which heart organoids could then be cultivated. These cardioids carry the patient's genetic mutations or specifically inserted gene mutations. This would allow researchers to observe directly in the lab what influence certain mutations have on the activity of genes or their switches, and how these affect heart muscle cells and heart rhythm. The model will therefore not only allow a better assessment of whether a genetic mutation is the cause of a heart disease but can also be used to develop possible therapies.
Are you also collaborating with other research groups on this project?
Yes, collaboration with other labs is a central and already very well-developed part of our project. Here at the University of Bern, and especially within our Cardiovascular Research Cluster, we benefit from a large and well-networked community with specialised labs in the field of cardiovascular research. Strong local collaborations already exist, for example with the group led by Katja Odening, which researches the causes and therapies of arrhythmias; with the team led by Nadia Mercader, a renowned expert in cardiac regeneration; and with the group led by Camilla Schinner, which specialises in arrhythmogenic cardiomyopathies.
The method is actively used and being further developed by several leading research groups in the field of cardiovascular research. Our cardioids have also played a central role in the success of the Lighthouse project at the Bern Center for Precision Medicine.
What hurdles need to be overcome before the methods can be implemented more widely?
In principle, the method is easy to learn if the necessary expertise and infrastructure are available. However, funding is a major challenge as this research is quite cost-intensive. In addition, we are dependent on the quality and availability of the biological source material. Another challenge is the natural variability of these human models compared to in vivo animal models. However, this can also be seen as an opportunity since biological diversity can be better modelled based on iPSCs from patients.
Who are the most important stakeholders in the project?
The most important stakeholders are primarily in the research sector. In Bern, there is a network with numerous research groups dedicated to cardiovascular topics. There are also close contacts with the clinic, where co-applicant Christian Zuppinger, among others, worked for many years, as well as links to the pharmaceutical and medical technology industry, which are supporting the project.
You are using artificial intelligence in the project. How does this technology support your research?
In the project, artificial intelligence is primarily used for the prediction of gene regions. A partner group from Vienna uses machine learning to generate precise predictions from large amounts of data about which genes are functionally relevant, without the need for complex experiments.
The aim is to use these AI-based predictions to significantly reduce the use of animal experiments by making experimental models more targeted and efficient. The field is developing very quickly and offers great potential for further improving research and disease models.
The output data we generate is also fed into the AI analyses, creating a valuable cycle of data and predictions.
